What Is the Molar Mass of Silicon Dioxide
How many grams of CO gas are made. 118g 1 mole Si 280855g 042015 moles Si.
![]()
Sio2 Lewis Structure Step By Step Construction What S Insight
In many parts of the world silica is the major constituent of sandSilica is one of the most complex and most abundant families of materials existing as a compound of several minerals and as a synthetic product.
. This compound is also known as Silicon Dioxide. When 152 g. Since there are two O atoms in one molecule of SiO2 the calculation will be.
The first thing you need to do here is to use the molar mass of silicon to convert your sample from grams to moles. The atomic mass of silicon dioxide SiO2 is 281 2160 601Amount of SiO2 mass of pure samplemolar mass 11601 0183mol There are 0183 moles of SiO2 in a 11 gram pure sample. Molar mass is the mass of one mole in grams of a given substance.
6 How many atoms are in sulfur. SiO2s 3Cs SiCs 2COg A. Silicon IV oxide sputtering target 508mm 20in dia x 318mm 0125in thick.
5 What is the mass of an atom of sulfur. 1 Show answers Another question on Chemistry. The density of silicon dioxide is 2648 gcm3.
Silicon carbide is produced by heating silicon dioxide and carbon at high temperatures as shown by the reaction below. The melting point of silicon dioxide is 1713 C. 1 g m o l.
601 Appearance white powdery substance solid when pure Density. 1650 75 C Boiling point. The SI base unit for amount of substance is the mole.
The molar mass of silicon dioxide is 6008 gmol. 7 What is molar mass of Sulphur. In this case the sample will contain.
When 500g of silicon dioxide is heated with an excess of carbon 322 g of silicon carbide Id produced. There are 2 oxygen atoms so we will multiply the mas. Silicon has a molar mass of 280855 g mol1 which means that 1 mole of silicon has a mass of 280855 g.
If a neutron-absorbing material is mixed in. The atomic mass of O is 159994 g. What is the percent yield of this reaction.
What is the mass of a mole of silicon dioxide. Molar mass of SiO2 is 6008430 000090 gmol Compound name is silicon dioxide. 4 What is the mass of 6022 x10 23 atoms of sulfur.
Americium-241 undergoes fission to produce three neutrons per fission event. What material does not melt at any temperature. This results in the units of gmol for molar mass.
The compound is insoluble in water or acids except hydrofluoric acid. What mass of carbon is required to react completely with 1570 g of SiO2. 60 g mole SiO2Explanation.
The molar mass of silicon dioxide S i O 2 2 8. 10 What is the average mass of a single sulfur atom in grams. What is the molar mass of silicon dioxide.
Tetrahedral Hazards NFPA 704. 8 How do you find the mass of 1 mole. The boiling point of silicon dioxide is 2950 C.
11 What is the mass of 121 x10 20 atoms of sulfur. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. The molar mass of octane is 11433 gmole.
350 mol 6009 gmol 210. 159994 x 2 280855. Surface area 175-225 m2g BET 998 trace metals basis.
Silicon dioxide is a transparent tasteless crystals found in nature as agate amethyst chalcedony cristobalite flint sand QUARTZ and tridymite. Download guide free counselling. There are 2 oxygen atoms so we will multiply the mass of oxygen by 2.
The number of grams is determined by adding up the atomic masses of the individual elements on the periodic table. Molar mass of SiO2 600843 gmol. Therefore the mass of a mole of Si is 280855 g 2 15999 g 600835 g.
You can view more details on each measurement unit. 2230 C Solubility in water 0012 g in 100g Structure Molecular shape. 9 How do you find the mass of moles.
Calculate the amount of grams of carbon dioxide formed from 100 g of octane. Molecular weight of SiO2 or mol This compound is also known as Silicon Dioxide. 1 2 1 6 6 0.
Molar mass of SiO2 600843 gmol. How many grams of SiC can be formed by reacting 200g of silicon dioxide and 200 grams of carbon. 1 1 0 0 4 7.
The percentage by mass of silicon in a sample of silicon dioxide 6 0. O 16 x 2 60 g mole SiO2. Silicon dioxide also known as silica is an oxide of silicon with the chemical formula SiO 2 most commonly found in nature as quartz and in various living organisms.
The mass of Si silicon is 28 and the mass of O oxygen is 16. Silicon dioxide Other names Silica Quartz sand Identifiers CAS number. 3 rows Silicon Dioxide molecular weight.
SiO2 molar mass6009 gmol. The mass of Si silicon is 28 and the mass of O oxygen is 16. The mass of a mole of silicon is 280855 g and the mass of a mole atomic oxygen is 15999 g.
1 grams SiO2 is equal to 0016643282854256 mole. The molar mass of SiO2 silicon dioxide is. Silicon dioxide nanopowder 10-20 nm particle size BET 995 trace metals basis.

Webelements Periodic Table Silicon Silicon Oxide
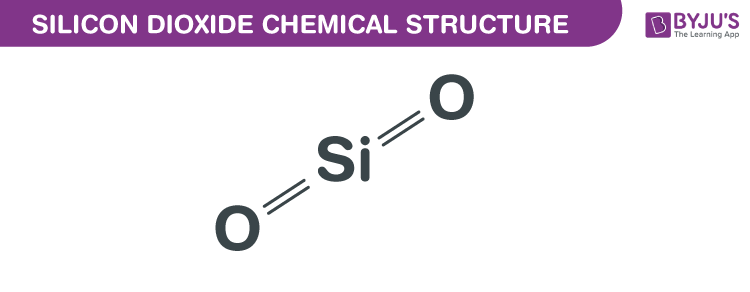
Silicon Dioxide Formula Chemical Structure Properties And Uses
Silicon Dioxide O2si Chemspider

Sio2 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
![]()
The Mole Just As A Is Always A Is Always Ppt Download
![]()
Covalent Calculations Ppt Download
![]()
Silicon Dioxide Alchetron The Free Social Encyclopedia
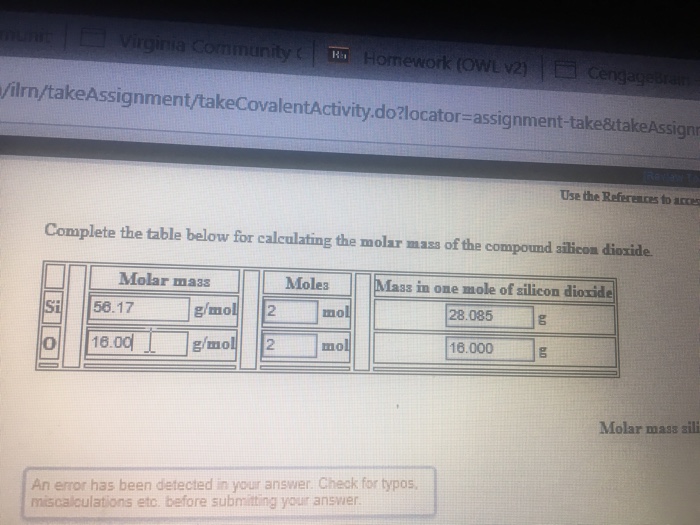
Solved Complete The Table Below For Calculating The Molar Chegg Com
![]()
Sio2 Lewis Structure Step By Step Construction What S Insight

8 Answer The Following Questions Using The Following Equation
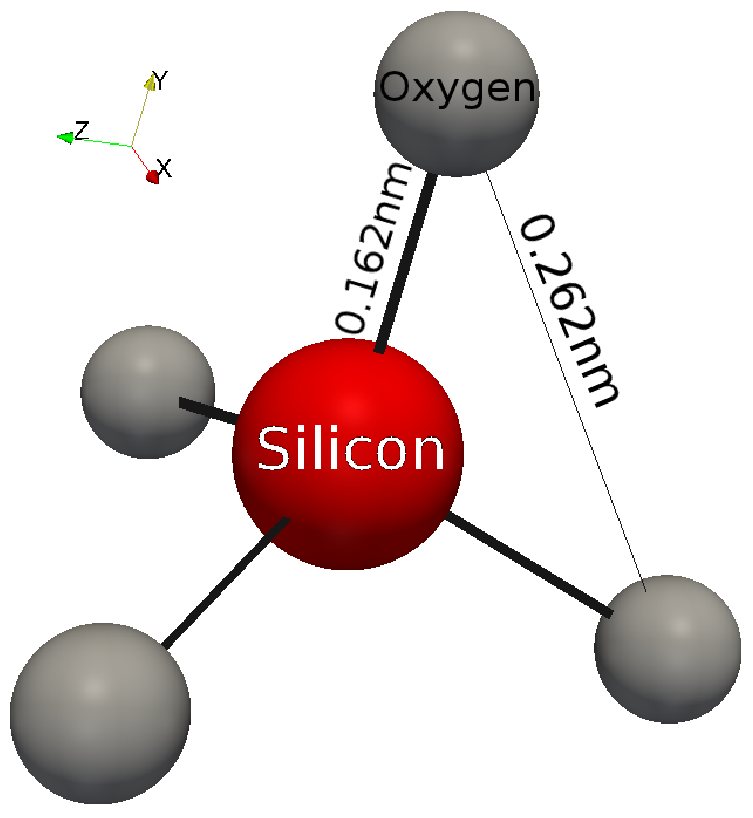
2 1 Silicon Dioxide Properties

How To Write The Formula For Silicon Dioxide Youtube
![]()
Silicon Dioxide Structure Properties Uses Of Sio2
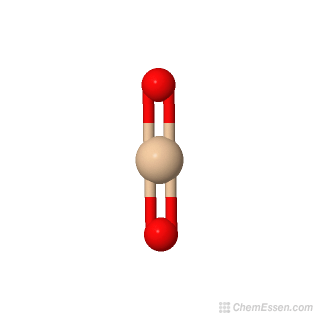
Silica Molecular Weight O2si Over 100 Million Chemical Compounds Mol Instincts
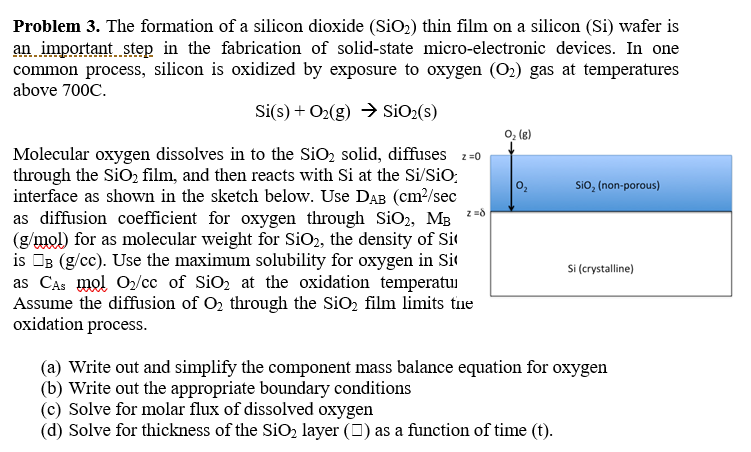
Problem 3 The Formation Of A Silicon Dioxide Sio2 Chegg Com

Molar Mass Calculations Mr Pauller Youtube

How To Write The Formula For Silicon Dioxide Youtube

Solved 59 List The Steps You Would Take To Calculate The Chegg Com
Comments
Post a Comment